Equipo de investigadores de la usach y de la Universidad Austral de Chile trabajó durante dos años en proyecto, con apoyo del Instituto Antártico de Chile, Inach.
Inducción de la enzima hemoxigenasa-1, que previene el daño en las vías respiratorias ocasionadas por la inflamación, podría ser utilizada como terapia. Director del Instituto Milenio de Inmunología e Inmunoterapia, IMII, dirige estudios sobre este virus, que es principal causa de infecciones severas del tracto respiratorio inferior en niños.
El desarrollo de nuevas drogas antivirales profilácticas y terapéuticas contra el virus respiratorio sincicial (VRS) es fundamental para controlar la carga de enfermedad en la población susceptible. Por ello, el Dr. Alexis Kalergis, académico de la Universidad Católica de Chile y director del Instituto Milenio de Inmunología e Inmunoterapia, IMII, examinó los efectos de una enzima, llamada hemoxigenasa-1 (HO-1), en la inflamación pulmonar inducida por este virus.“Los resultados de nuestros estudios muestran que después de la infección por VRS, la aplicación de HO-1 disminuyó la replicación viral e inflamación pulmonar de los modelos de análisis transgénicos. Además, observamos efectos antivirales y protectores similares. Finalmente, los datos in vitro sugieren que la inducción de esta enzima puede modular la susceptibilidad de las células a esta infección, especialmente las de tipo epiteliales, de las vías respiratorias”, señaló el académico.
Esta investigación puede complementar a la vacuna contra la enfermedad, desarrollada en nuestro país por el bioquímico, que se ha posicionado a nivel mundial con sus investigaciones y resultados favorables frente a esta afección, una de las que provoca mayor hospitalización y fallecimiento en menores de dos años. La aplicación de su antídoto ha mostrado seguridad y entregado resultados exitosos en un primer grupo de voluntarios.
La enzima se encuentra de forma natural en el organismo, expresada en muchas células y tejidos, sobretodo en el bazo, riñón e hígado. Sin embargo, también han descubierto que en ciertas patologías, sus niveles están disminuidos. Por esta razón, han podido explicar que la deficiencia de HO-1 en el organismo se asocia a un perfil “inflamatorio importante, y el desarrollo de patologías inflamatorias y otras de carácter autoinmune, como diabetes tipo I y Lupus”, afirma el Dr. Kalergis.
Los científicos del IMII llevan más de una década explorando la acción terapéutica de HO-1. Al respecto, el Dr. Kalergis señala que la enzima tiene capacidad para controlar la función de las células dendríticas, las cuales se encuentran desreguladas en pacientes con problemas de autoinmunidad, promoviendo una sobrerreacción en la respuesta inmune. ¿Cuál es el secreto de la enzima? Lo novedoso es que ésta libera pequeñas dosis de un gas responsable de la función antiinflamatoria. “Este gas funciona como un inductor de tolerancia. Y no es tóxico, ya que se libera en cantidades reducidas al interior de las células, y no a nivel sistémico. Su acción se da en la mitocondria, estructuras que controlan la cantidad de energía, y al hacerlo, las células bajan su nivel energético, lo que a su vez disminuye la actividad inflamatoria, fomentando así la prevención”, comenta el científico.
Virus respiratorio
El virus respiratorio sincicial, es de alta incidencia en todo el mundo. Principalmente en los inviernos y favorecido por el frío, contaminación y humedad, este microorganismo ocasiona bronquitis obstructiva, infecciones de vías respiratorias altas, así como neumonía en los casos más severos.
En Chile, el Estado invierte sobre 10 mil millones de pesos anuales en tratamientos, hecho que se suma al colapso de los sistemas hospitalarios. Por estas razones, el doctor en microbiología e inmunología estima que contar con la vacuna en el mercado “será de gran impacto en la comunidad afectada, en sus familias y también traerá beneficios desde el punto de vista económico, ya que permitirá prevenir los daños”.
Considerando que la mayor vulnerabilidad de contagio por el virus, ocurre entre los 0 y 2 años, la estrategia es poder aplicar el antídoto a las pocas horas de nacimiento del bebé. “El blanco principal serán los infantes y pensamos que con una sola dosis bastaría. Nuestra intención es llegar a reemplazar la actual vacuna de BCG, contra la tuberculosis y que la nuestra genere protección contra ambos patógenos”, señala el bioquímico de la Universidad Católica.
Patentes internacionales
En 2017, la vacuna recibió la concesión de la patente china. El profesor titular de la Universidad Católica de Chile, celebró este vínculo con el país asiático, gracias al cual “la vacuna ya cuenta con protección intelectual en esta nación, lo que abre paso a su comercialización y uso en beneficio de millones de personas”.
El antídoto también fue patentado en Estados Unidos el año 2013, con apoyo de un proyecto FONDEF-Interés Público, adjudicado por el científico y su laboratorio “Este hecho representa una contribución importante a resolver un problema de gran significancia para la salud pública mundial”, comenta el Dr. Kalergis.
Su trabajo está orientado a nivel molecular en reestablecer el equilibrio inmunológico en fenómenos autoinmunes. Gracias a la generación de la vacuna contra el virus respiratorio sincicial ha recibido reconocimientos internacionales como la Medalla de Oro para inventores que entrega la Organización Mundial de la Propiedad Intelectual (OMPI). El galardón, el cual se entrega desde 1979, destaca la importancia de los más de diez años de estudios del profesor titular de la Universidad Católica en relación a encontrar una vacuna para el VRS.
Además, realiza investigaciones enfocadas al entendimiento de los mecanismos moleculares responsables de la regulación de la sinapsis inmunológica y desarrollo de enfermedades autoinmunes, tales como la Esclerosis Múltiple, el Lupus Eritamatoso Sistémico y la Artritis Reumatoide.
El director del Instituto Milenio en Inmunología e Inmunoterapia, es Bioquímico de la Pontificia Universidad Católica de Chile y Doctor en Microbiología e Inmunología del Albert Einstein College of Medicine en Nueva York–USA, donde posteriormente realizó un post-doctorado.
Actualmente se desempeña como profesor e investigador en el Departamento de Genética Molecular y Microbiología de la Facultad de Ciencias Biológicas y en la Facultad de Medicina de la Universidad Católica de Chile.
A lo largo de su carrera ha publicado numerosos artículos científicos en revistas especializadas de alto impacto y contribuido en la formación científica de decenas de estudiantes de pre- y postgrado en el área de la inmunología.
Fuente: www.elmostrador.cl
Esto se debe a que “estamos acostumbrados desde el punto de vista evolutivo a funcionar de día y descansar de noche”.
Incluso, “aquéllos que son ordenados en comer” en términos de horario, “no están subiendo tanto” de peso, expuso el especialista de la UC.
Larrondo recomendó evitar ver televisión, celulares o tablets de noche porque se genera trastornos en el sueño.
Date: January 4, 2018
Source: UT Southwestern Medical Center
Summary: Researchers have used precision editing of the bacterial populations in the gut to prevent or reduce the severity of inflammation in a mouse model of colitis.
UT Southwestern Medical Center researchers have used precision editing of the bacterial populations in the gut to prevent or reduce the severity of inflammation in a mouse model of colitis.
The potential strategy — which targets metabolic pathways that are active only during intestinal inflammation — prevented or reduced inflammation in a mouse model of colitis while exerting no obvious effect in control animals with healthy, balanced bacterial populations, said Dr. Sebastian Winter, Assistant Professor of Microbiology and co-corresponding author of the study published online today in Nature.
“Our results provide a conceptual framework for precisely altering the bacterial species that line the gut in order to reduce the inflammation associated with the uncontrolled proliferation of bacteria seen in colitis and other forms of inflammatory bowel disease [IBD],” he said.
“We stress that this is a proof-of-concept study in which a form of tungsten, a heavy metal that is dangerous in high doses, was used. It is never safe to ingest heavy metals. Now that we have a drug target [the bacterial pathway], our goal is to find a safe therapy that exerts a similar effect,” added Dr. Winter, a W.W. Caruth, Jr. Scholar in Biomedical Research at UT Southwestern.
Like plants in a garden, the diverse populations of microbes that normally line the intestinal tract, called the microbiota, are essential to human health. They aid in digestion, educate the immune system, and fend off infections. However, when the microbial populations become unbalanced, these otherwise beneficial bacteria become a liability, similar to garden plants that become invasive and push out competing species, he explained.
One of the main hurdles in understanding the biology of the gut microbiota is its vast diversity. In humans, hundreds of different species of bacteria are found in the intestinal tract, and the composition of species varies remarkably between individuals.
Changes in the composition of the gut microbiota are seen in many human diseases such IBD, a chronic, lifelong inflammatory disorder that includes Crohn’s disease and ulcerative colitis. The Centers for Disease Control and Prevention estimates that at least 1 million adults in the United States are affected by IBD. The condition currently has no cure or prevention. Changes in the gut microbiota also occur in Type 2 diabetes, colon cancer, HIV-related intestinal disease, and the necrotizing enterocolitis seen in certain preterm infants, Dr. Winter said.
Some of the bacteria in the gut microbiota that are linked to inflammatory diseases are those in the Enterobacteriaceae family. Members of that family, including nonpathogenic E. coli (Escherichia coli), are present in small numbers in the healthy gut and protect against infection with pathogens such as Salmonella, a common cause of food poisoning.
However, in IBD patients and in mouse models of colitis, Enterobacteriaceae species grow uncontrollably, said Dr. Wenhan Zhu, co-lead author and a postdoctoral researcher in the Winter laboratory.
In recent work published in Cell Host & Microbe, the Winter laboratory reported that the way members of the Enterobacteriaceae family generate cellular energy for growth and obtain nutrients differs from other gut bacteria. They appear to use unique metabolic tricks to fuel their overgrowth and to push out competing beneficial gut bacteria during illness.
“These pathways are unique in the sense that they are only present in certain bacteria and only function during gut inflammation. That situation presented an opportunity for rational design of prevention and treatment strategies for conditions related to gut inflammation, such as IBD,” Dr. Winter explained.
That observation led to the current study in Nature, which used a form of the heavy metal tungsten to inhibit the pathogen’s metabolic tricks.
“The overall idea is that the tungsten threw a wrench into the way Enterobacteriaceae produce energy, slowing the growth of the pathogenic bacteria during flares of inflammation,” said Dr. Zhu.
The researchers found that tungsten is taken up by bacteria and inadvertently incorporated into an important bacterial cofactor. The resulting poisoned cofactor does not function properly and derails the ability of Enterobacteriaceae to generate energy in the inflamed gut. In mouse models, oral administration of tungstate, a soluble tungsten salt, in the drinking water selectively prevented the bloom of Enterobacteriaceae in the gut, they said. Nearby beneficial bacteria were unaffected, apparently because their energy-generating metabolism does not rely on that particular cofactor.
“It is worth noting that our strategy only inhibits the bloom of Enterobacteriaceae during intestinal inflammation without getting rid of them entirely. This finding is important because in the proper ratios, Enterobacteriaceae also fulfill the role of resisting colonization by bacterial pathogens,” Dr. Winter said. “Therefore, controlling the bloom of these bacteria during episodes of inflammation is preferable to removing them from the system completely.”
Although experimental evidence is scarce, it has long been speculated that changes in the gut microbiota composition can worsen disease, Dr. Winter said.
In this study, tungstate treatment in mouse models of colitis shifted gut microbiota to a more normal state in terms of the balance of bacterial species and also reduced gut inflammation, the researchers report. Tungstate treatment did not cure the disease, but it improved the overall health of the animals.
“We only used tungsten in ‘proof-of-concept’ experiments to identify a potential molecular target, and we are still far from turning this basic discovery into a therapeutic treatment in patients,” Dr. Winter said. Exposure to tungsten — a heavy metal — can potentially have serious negative effects, such as neurological and reproductive harm, he added.
Traditional therapeutic approaches focus on treating the human host. But these latest results give hope that, in principle, it may be possible to harness normal gut bacteria to achieve a positive outcome for the host, for example by carefully steering the function and composition of the gut microbiota during gut inflammation, Dr. Winter explained.
“When doctors use broad-spectrum antibiotics, the goal is to kill off as many bacteria as possible. If a patient shows up in a clinic very ill and there is no time to identify a specific pathogen, broad-spectrum antibiotics will be used,” Dr. Winter said. “The effects of broad-spectrum antibiotics on the gut microbiota are devastating. It’s like using a torch in a flower bed and hoping that once you kill the weeds, the flowers will flourish.
“In our case, we found a way to target only one family of bacteria, the Enterobacteriaceae, and only during inflammation,” he said. “More study is needed to find potential therapies for human disease, but this is a promising first step.”
UTSW co-authors include: Co-lead author Maria Winter, a research associate; Dr. Luisella Spiga, a postdoctoral researcher; visiting fellow Lisa Büttner; graduate students Elizabeth Hughes and Caroline Gillis, all of Microbiology; Dr. Breck Duerkop, Instructor, Immunology; Cassie Behrendt, a research technician, Immunology; Dr. Lora Hooper, Professor and Chair of Immunology with appointments in Microbiology and in the Center for the Genetics of Host Defense, a HHMI Investigator and holder of the Jonathan W. Uhr, M.D. Distinguished Chair in Immunology, and the Nancy Cain and Jeffrey A. Marcus Scholar in Medical Research, in Honor of Dr. Bill S. Vowell; Dr. Luis Sifuentes-Dominguez, Instructor of Pediatrics; Dr. Kayci Huff-Hardy, clinical fellow, Internal Medicine in the Division of Digestive and Liver Diseases; Dr. Andrew Koh, Associate Professor of Pediatrics and Microbiology and in the Harold C. Simmons Comprehensive Cancer Center as well as Director of Pediatric Hematopoietic Stem Cell Transplantation at Children’s Health; and Dr. Ezra Burstein, Professor of Internal Medicine and Molecular Biology and Chief of the Division of Digestive and Liver Diseases. Researchers from the University of California, Davis and Temple University in Philadelphia also participated.
The study was supported by National Institutes of Health Public Health Service grants, The Welch Foundation, the HHMI, the American Cancer Society, and the Crohn’s and Colitis Foundation. The funders had no role in study design, data collection, or interpretation.
Story Source:
Materials provided by UT Southwestern Medical Center. Note: Content may be edited for style and length.
Journal Reference:
- Wenhan Zhu, Maria G. Winter, Mariana X. Byndloss, Luisella Spiga, Breck A. Duerkop, Elizabeth R. Hughes, Lisa Büttner, Everton de Lima Romão, Cassie L. Behrendt, Christopher A. Lopez, Luis Sifuentes-Dominguez, Kayci Huff-Hardy, R. Paul Wilson, Caroline C. Gillis, Çagla Tükel, Andrew Y. Koh, Ezra Burstein, Lora V. Hooper, Andreas J. Bäumler, Sebastian E. Winter. Precision editing of the gut microbiota ameliorates colitis. Nature, 2018; DOI: 10.1038/nature25172
Source: www.sciencedaily.com
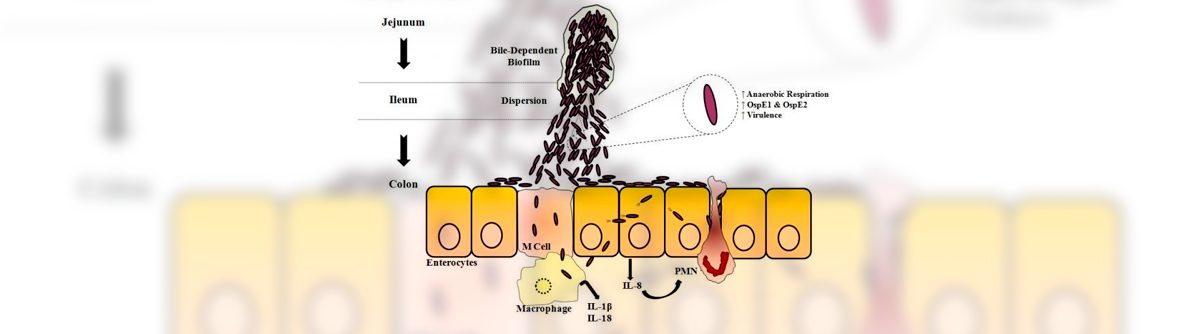
Exposure to bile salts leads to formation of protective biofilms as pathogen passes through small intestine
- Date: May 31, 2017
- Source: Massachusetts General Hospital
- Summary: Researchers have discovered how the bacteria Shigella survives its journey from the mouth to the colon, taking advantage of substances that would kill many less persistent organisms.
Surviving the treacherous journey through the human body from the mouth to the colon takes a special kind of bacterial pathogen. Shigella — a group of bacteria responsible for much of the diarrheal disease affecting children in the developing world — travels unimpeded from the mouth to the colon, where they unleash powerful machinery to trigger debilitating diarrhea. Researchers from Massachusetts General Hospital (MGH) have been looking not only at how Shigella survives this journey but also how it takes advantage of substances that would kill many less persistent organisms. Each year Shigella is responsible for at least 80 million infections and approximately 700,000 deaths worldwide. Long-term effects for Shigella survivors can include impaired physical and cognitive development, poor gastrointestinal health, reactive arthritis or kidney damage depending on the strain causing infection. Although 99 percent of cases occur in developing nations, approximately half a million occur in the U.S. each year.
To gain important insights into the pathogenesis of Shigella, MGH researchers focused on its mechanisms of virulence and survival as the organism travels to the colon. Among other findings, they determined that Shigella uses multiple mechanisms to survive exposure to bile salts in the small intestine. An essential component of digestion, bile destroys many harmful bacteria, but it cannot disarm intestinal pathogens such as E. coli, Salmonella, Vibrio and Shigella.
“For the first time, we have identified how Shigella not only resists bile but also uses this alkaline fluid produced by the liver to its advantage,” says Christina S. Faherty, PhD, of the Mucosal Immunology and Biology Research Center (MIBRC) at MGH, senior author of a paper published in the June issue of Infection and Immunity. “We analyzed how the pathogen’s gene expression changes in response to bile salts exposure. The changes we identified pointed to the use of antibiotic resistance mechanisms to resist bile, to the development of a more infectious organism through increased virulence gene expression, and to one better able to survive the colonic environment due to additional gene expression changes.” Subsequent mutational analyses confirmed the bile resistance mechanisms of Shigella.
With no current vaccine against Shigella, antibiotics are the only treatment option. But like so many pathogens, Shigella has developed resistance to many antibacterial drugs. “The ability of Shigella to resist antibiotics so efficiently may be partly due to the bacteria’s exposure to bile during transit of the small intestine,” says Faherty. “It appears that bile primes intestinal pathogens for antibiotic resistance, since many of the same mechanisms used to resist bile exposure are used to resist antimicrobials. Our findings on Shigella’s bile resistance mechanisms could have important implications for overcoming multi-drug resistance.”
The study also highlighted an additional response of Shigella to bile. Previous work by Faherty and other researchers has shown that two hours of exposure to bile salts increases the ability of Shigella to adhere to and invade epithelial cells lining the gastrointestinal tract. By prolonging the exposure to mimic the time required for Shigella to transit the small intestine, Faherty’s current work demonstrated for the first time that longer exposure to bile salts led to the formation of biofilms — communities of bacteria that produce a protective coating to resist harsh environmental conditions.
Faherty believes biofilm formation enables Shigella to clump together to transit through the small intestine. Faherty’s team also found that the reabsorption of bile salts that normally takes place in the lower small intestine causes the biofilm to disperse, releasing the hyper-virulent bacteria to infect tissues in the colon. In all, these observations provide a more complete picture of how Shigella transits the small intestine to reach the colon for infection.
An assistant professor of Pediatrics at Harvard Medical School, Faherty is enthusiastic about the study’s insights into Shigella pathogenesis and hopes this research could lead to new strategies to combat antibiotic resistance and develop vaccines. “Researchers have been trying to find a successful candidate vaccine to fight Shigella for more than 50 years,” she says. “By identifying some of the early mechanisms of how Shigella navigates the intestine and demonstrating how the bacteria use bile as a signal to prepare for infection in the colon, we now have a greater understanding for developing potential new therapies.”
Story Source:
Materials provided by Massachusetts General Hospital. Note: Content may be edited for style and length.
Source: www.sciencedaily.com
For the human body to mount an immune response to a viral infection, host cells must identify the viral invader and trigger a signaling pathway. This signal then prompts the immune system to attack and subdue the pathogen. Using the dengue virus (DENV) as a model, researchers from the Icahn School of Medicine at Mount Sinai have identified the “viral sensor” that initiates an immune response and have also described how the virus counteracts this mechanism and evades immune detection. The paper describing these findings was published in the journal Nature Microbiology.
Along with aiding in the design of future vaccines, understanding how host cells signal the need for an immune response and the sophisticated mechanisms viruses use to avoid recognition can illuminate patient susceptibility to disease severity. It can also inform techniques to dampen unwanted pro-inflammatory responses associated with autoimmune diseases.
“Previous studies have shown that human viruses have acquired specific mechanisms to strategically avoid detection by the innate immune system. Active strategies are used by viruses to minimize the ability of cells to detect and respond to infection, allowing sufficient time for the production of viral progeny,” said last author of the study Ana Fernandez-Sesma, PhD, Professor, Microbiology, Icahn School of Medicine at Mount Sinai. “Our study shows how dengue virus, which affects people around the globe, employs multiple techniques to avoid detection. We shed light on the mechanisms cells use to recognize the traces of viral infection within a cell and the methods viruses have acquired to obstruct them. It is this recognition that eventually leads to an immune response.”
Researchers identified cyclic GMP-AMP synthase (cGAS) as the protein responsible for initially detecting viral infection. cGAS, a cytosolic DNA sensor, recognizes DNA that has escaped the nucleus or mitochondria of a cell and entered the cytoplasm, an unusual occurrence. In the case of DENV infection, cGAS recognizes traces of mitochondrial DNA released into the cytoplasm as a consequence of the beginning stages of the infection; it does not recognize the viral particles themselves. Once cGAS binds to DNA, it activates a series of cascading chemical triggers known as the cGAS/cGAMP/STING sensing pathway, which induces type I interferon (IFN) signaling and begins the immune response. Although cGAS has been characterized as a DNA sensor, it has antiviral properties against different positive-strand RNA viruses, like DENV—a characteristic that has not yet been fully explored.
DENV in turn reduces the likelihood of triggering the cGAS/cGAMP/STING pathway by degrading cGAS and preventing it from binding with mitochondrial DNA in the cytoplasm of the cell. The DENV-encoded protease cofactor NS2B promotes cGAS degradation in an autophagy-lysosome-dependent mechanism. Previous research from this group has shown that DENV cleaves to STING, an endoplasmic reticulum resident host protein, to prevent type I IFN signaling. Uncovering the role DENV plays in degrading cGAS and stopping the preliminary step of the immune-signaling pathway confirms two separate but coordinated mechanisms the virus uses to thwart a host immune response.
The interplay between DENV and the mitochondria is a field of increasing interest, and by exploring that relationship this study describes a novel mechanism by which human cells can detect damage generated during the early stages of an infection. By releasing its genomic DNA inside the cell, the mitochondria initiate the cGAS/cGAMP/STING pathway, type I IFN signaling, and the immune response. However, DENV has learned to counteract this “maternal” protection mechanism by NS2B-induced degradation of cGAS.
“Mapping how cGAS recognizes DENV and the role mitochondrial DNA plays in creating an immune response is another novel insight of this study,” Dr. Fernandez-Sesma said. “Until now, it has not been understood how cGAS can play such a critical role in identifying these RNA viruses. Our data strongly suggest that mitochondrial damage and the release of mitochondrial DNA are intrinsic collateral damage during DENV infection and prompt cGAS to activate the necessary immune signaling pathways.”
DENV infects close to 400 million people every year, globally, and almost half of the world population lives in areas where the same mosquito species can transmit dangerous viruses like dengue, yellow fever and Zika, among others. Finding new ways to combat DENV and similar viruses can play a crucial role in lessening the enormous global health burden they represent. Further, charting the strategies viruses use to counteract the immune system can be used as a platform for the design of chemical compounds that can mimic this inhibitory effect and address the inflammatory process observed in many autoimmune diseases.
![]() Explore further: Researchers discover immune system’s ‘Trojan Horse’
Explore further: Researchers discover immune system’s ‘Trojan Horse’
More information: Sebastian Aguirre et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection, Nature Microbiology (2017). DOI: 10.1038/nmicrobiol.2017.37
It has become increasingly evident that, like animals, plants are not autonomous organisms but rather are populated by a cornucopia of diverse microorganisms.
Afew years ago, as a postdoc in the lab of Paul Schulze-Lefert at the Max Planck Institute for Plant Breeding Research in Cologne, Germany, I used next-generation sequencing to study the bacterial communities that populate roots of the model plant Arabidopsis thaliana. Although scientists had known for many years that roots interact with a variety of microorganisms, the composition of these communities was still poorly understood. As our sequencing data began rolling in, I was stunned by the staggering taxonomic diversity of bacteria that a single, tiny root can host. Yet there was an order in this apparent chaos. Almost invariably, members of the phyla Actinobacteria, Bacteroidetes, and Proteobacteria were enriched, differentiating the root specimens from the surrounding environment.
Subsequent studies by other labs supported our findings and posited Firmicutes as an additional dominant member of the plant microbiota. In addition to these bacterial groups, genomic surveys of plants have revealed certain fungal and eukaryotic microbes. And all of these groups of organisms are making themselves at home not just beneath the soil in and around plants’ roots, but in other tissues, such as leaves, as well.
This research immediately raised new questions: Why were certain microbes more abundant in roots and leaves? How did these microbial communities assemble? And most critically, how did they affect plant health?
Recently, in addition to genomic surveys of the microbes present in various plant tissues, researchers have begun to probe the functional consequences of these bacterial, fungal, and eukaryotic symbionts. A better understanding of the molecular dialog between plants and their microbiota could revolutionize agriculture. The world population is expected to reach 9.8 billion in 2050, more than 30 percent larger than at present. This will put enormous pressure on food production globally—pressure that won’t be relieved solely by the agrochemicals farmers currently use to increase yield and protect crops from pests and pathogens. To encourage a sustainable food source for humanity, radical changes in the crop production process are needed—changes that could come in the form of microbial manipulation.
The interface between plant roots and soil—a zone called the rhizosphere—and the root itself are sites of colonization for microbes capable of enhancing mineral uptake by the plant, of both actively synthesizing and modulating the plant’s synthesis of chemical compounds called phytohormones that modulate plant growth and development, and of protecting plants from soil-derived pests and pathogens. For these reasons, scientists are looking to manipulate the microbes populating this belowground habitat to sustainably increase crop production. And in my lab, we are looking at ancient varieties and wild relatives of crops as a source of insights into beneficial associations between plants and microbes that could be adapted for agricultural settings.
Surveying the plant microbiome
The roots of land plants thrive in soil, one of the richest and most diverse microbial reservoirs on Earth. It has been estimated that a single gram of soil contains thousands of different bacterial species, not to mention other microorganisms such as archaea, fungi, and protists. Perhaps not surprisingly, the establishment of interactions with the soil biota represented a milestone for plants’ adaptation to the terrestrial environment. Fossil evidence suggests that the first such interactions with fungal members of the microbiome occurred as early as ~400 million years ago.1

PLANTS’ MICROBIAL COMMUNITIES: Like animals, plants host communities of microbes that influence a wide variety of their biological processes. Recent surveys of the plant microbiome have begun to document which species are present—including not just bacteria, but fungi and microscopic eukaryotes as well—and how they affect the plant’s health and functioning.
See full infographic: WEB | PDF© MESA SCHUMACHER
Comparative studies indicate that soil characteristics such as nutrient and mineral availability are major determinants of the root microbiome. Just as digestive tract microbes interact with the food consumed by vertebrates, the root microbiome mediates the soil-based diet of plants. Also paralleling host/microbe interactions in the animal kingdom, individual members of the plant microbiome appear to be compartmentalized. I and other researchers working with Arabidopsis and with rice have identified at least three distinct microbiomes thriving at the root-soil interface: that in the rhizosphere; another one on the root surface, or rhizoplane; and a third one inside the root, an area known as the endosphere.2,3In all three compartments, Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria dominate the bacterial communities in multiple plant species. The aboveground portions of plants such as leaves show similarly predictable microbial composition. (See illustration at left.)
While the categories of microbes that make up the plant microbiome are largely conserved, much variation exists in the species compositions of these communities across hosts. One key factor in determining how the microbiome is populated and maintained appears to be the plant’s release of organic compounds into the rhizosphere, a process known as rhizodeposition. The amount and composition of these organic deposits vary depending on plant species and developmental stage, but may account for up to 11 percent of net photosynthetically fixed carbon and 10 percent to 16 percent of total plant nitrogen.4 This process influences the chemical and physical composition of the rhizosphere and, in turn, provides signaling molecules and organic substrates for microbial growth.

ROOT BUGS: Plant roots and the interface between the roots and the soil—a zone called the rhizosphere—are home to diverse microbes that can affect mineral uptake by the plant.© BIOPHOTO ASSOCIATES/SCIENCE SOURCE
Another factor that likely shapes the composition of the plant microbiome is interaction between microbes. In 2016, Eric Kemen of the Max Planck Institute for Plant Breeding Research and colleagues surveyed the microbes thriving in and on wild Arabidopsis leaves at five natural sites in Germany sampled in different seasons. They then plotted correlations between the abundances of more than 90,000 pairs of microbial genera identified in their survey, revealing six “microbial hubs”—nodes with significantly more connections than other nodes within the network. These hubs were represented by the oomycete genus Albugo, the fungal genera Udeniomyces and Dioszegia, the bacterial genus Caulobacter, and two distinct members of the bacterial order Burkholderiales.5 Given the high degree of connectivity within the communities, it is likely that these microbial hubs play a disproportionate role in the microbiome, akin to that of keystone species in an ecosystem.
To validate this idea that certain species can drive the composition of the plant microbiome, Kemen’s team selected Albugo sp. and Dioszegia sp. as paradigmatic examples of microbial hubs. Albugooomycetes are eukaryotic pathogens of Arabidopsis with an obligate biotrophic lifestyle—meaning that they cannot be cultured outside their host. Consistent with the central role of Albugo in the plant’s microbial community, Arabidopsis that had been artificially infected with Albugo laibachii and maintained in potting soil under controlled conditions displayed a bacterial microbiome composition that was less variable across plants than that of uninfected individuals. Conversely, differences between the bacterial microbiomes of three distinct Arabidopsis strains were amplified in the presence of A. laibachii infection. The fungal microbiome, however, was not significantly affected by the presence of A. laibachii and another Albugo species.
Kemen’s team conducted a parallel set of experiments with Dioszegia sp., which—unlike Albugo sp.—are culturable under laboratory conditions, and six bacterial isolates from Arabidopsis leaves. The results confirmed that the presence of the fungal species can strongly inhibit the growth of Caulobacter—plants whose leaves were inoculated with Dioszegia sp. showed a 100-fold reduction in the number of colony-forming units of Caulobacter sp.—mirroring the significant negative correlation observed between these two groups of microbes in the network analysis.5
In 2017, Harvard University’s Roberto Kolter and colleagues demonstrated that such microbial interactions are not limited to Arabidopsis. The researchers developed a simplified version of the maize root microbiome, consisting of seven bacterial strains previously identified in sequencing surveys. By using a leave-one-out approach to colonizing naive maize plants, they demonstrated that removal of Enterobacter cloacae disrupts the composition of the microbial community, which became dominated by Curtobacterium pusillum, while the other five species had nearly disappeared. Interestingly, this effect was limited to plant colonization: when the seven strains of bacteria were monitored in a substrate that did not contain maize seedlings, the community’s composition was significantly different from the one retrieved from roots, and the regulatory role exerted by E. cloacae was not detected.6
These studies suggest that individual members of the microbiome can have a disproportionate role in assembling and stabilizing the community. Deciphering the interactions within and between the various taxa populating leaves and roots will be required to understand the regulation of the plant microbiome.
From composition to function
For years, researchers have observed that, despite the presence of pathogens and conditions favorable to infection, some regions produce plants that are less susceptible to disease than other areas. The soils in these areas, it turns out, support plant health via the microbiome.
Researchers are making strides in understanding the mechanisms underlying this support. In 2011, for example, a team led by Rodrigo Mendes, then at Wageningen University and Research Centre in the Netherlands, demonstrated that disease suppression was linked to the recruitment of a specific population of Pseudomonadaceae, a family of the phylum Proteobacteria. Using a PCR fingerprinting approach, the researchers discerned that this population could be grouped into ten haplotypes, which the team designated A to J. Of these, haplotypes A, B, and C represented some 90 percent of the isolated bacteria. When inoculated in soil, a representative strain of haplotype C suppressed the incidence of disease caused by the fungus Rhizoctonia solani on sugar beet roots, while, surprisingly, strains from haplotypes A or B did not.7
Similarly, in their study published last year, Kolter and colleagues found that maize plants inoculated with the seven selected bacterial strains showed significantly delayed development of Fusarium verticillioides, the causal agent of maize blight. This phenomenon was mediated by the specific strains chosen, and not by bacterial colonization per se, as seed treatment with a laboratory strain of Escherichia coli did not protect maize seedlings from pathogen development. Likewise, the seven strains together were required for the protective effect: inoculation with individual strains resulted in significantly less protection against F. verticilloides.
This method of combining sequencing data with microbial isolation is becoming a powerful tool to formulate testable hypotheses and gain novel insights into the function of the plant microbiome. Like Kolter, researchers are assembling microbial isolates into synthetic communities (SynComs) of known composition and testing their effects on host plants. This approach was once considered a daunting task, as only a very limited fraction—often less than 1 percent—of soil biota was considered culturable under laboratory conditions. But in 2015, Schulze-Lefert’s lab teamed up with Julia Vorholt’s group at ETH Zurich in Switzerland to investigate the proportion of Arabidopsis-associated bacteria that can be cultured, and found the 1 percent statistic to be a vast underestimate.

FUNGAL FINGERS: In addition to bacteria, the plant microbiome includes fungal species such as the Rhizoctonia solani shown here.© DENNIS KUNKEL MICROSCOPY/SCIENCE SOURCE
Comparing the taxonomic relationships among some 8,000 colony-forming microbes from leaves and roots of plants using cultivation-independent sequencing surveys of leaf and root microbiomes, the researchers demonstrated that more than 50 percent of the dominant members of the Arabidopsismicrobiome can be cultured in vitro.8 Taking advantage of this finding, the team assembled SynComs representative of the microbiota of the Arabidopsis roots and leaves and tested the communities’ capacities to colonize these tissues on plants grown in a sterile substrate—the botanical equivalent of germ-free mice. These experiments revealed that, upon plant inoculation, root and leaf isolates form microbial communities resembling the natural microbiomes of those tissues, demonstrating that the SynCom approach accurately recapitulates the effects of a complete microbiota.8
Since then, numerous researchers have begun to develop SynComs to further explore the function of the plant microbiome. Earlier this year, for example, Jeff Dangl of the University of North Carolina at Chapel Hill and colleagues used the SynCom approach to explore the role of the root microbiome in phosphate uptake. In nature, less than 5 percent of the phosphorus content of soils is available to plants. To circumvent this limitation, farmers rely on the application of chemical fertilizers, but this approach is not sustainable in the long term. Thus, understanding how plants and their associated microbes can thrive under sufficient and limiting phosphorus supplies is a priority. There is a huge body of literature documenting the contribution of arbuscular mycorrhizal fungi to phosphorus uptake in plants, but the role of the bacterial microbiota remains mysterious.
In experiments with Arabidopsis, which does not engage in symbiotic relationships with mycorrhizal fungi, Dangl and his colleagues compared the microbiomes of wild-type plants with those of mutant lines that had impaired phosphate starvation responses (PSRs)—a set of morphological, physiological, biochemical, and transcriptional activities evolved by plants to cope with phosphorus deficiency. Using a SynCom represented by 35 taxonomically diverse bacterial isolates from Arabidopsis and related plants, the researchers demonstrated that wild-type plants and mutants, grown on agar plates, assemble distinct root communities when exposed to both low and high phosphorus concentrations. Remarkably, SynCom inoculation reduced accumulation of phosphorus when plants were grown under limited conditions but not when plants were grown in the presence of abundant phosphate, suggesting that bacteria and plants compete for the element.
By monitoring a core set of 193 marker genes, the team observed that SynCom inoculation greatly enhanced PSR-related transcription in wild-type plants. When the researchers transferred inoculated wild-type plants grown with limited phosphorus to plates with sufficient supplies, they observed a striking result: 20- to 40-fold increases in phosphorus concentration in the plant stem, as compared with mock-inoculated controls. Such a dramatic increase in phosphorus uptake was not detected in inoculated plants initially grown with sufficient phosphorus. Therefore, initial plant-bacteria competition for phosphorus might be part of an adaptive mechanism to maximize PSR in plants.9
Further investigation into the binding sites of transcription factors on Arabidopsis DNA revealed that PHR1, a master regulator of PSR, and its paralog PHL1 contribute to transcriptional regulation of plant immunity. In particular, phr1;phl1 mutant plants display enhanced activation of plant immunity genes in response to phosphate starvation and to SynCom inoculation, compared with wild-type plants. Together, these data suggest that the nutritional status of the host is a driver of microbiome composition; through master regulators of mineral starvation, plants can modulate immune responses, which could, in turn, shape microbiome composition. (See “Holding Their Ground,” The Scientist, February 2016.)
What’s next?
Characterizing the plant microbiome and its function could be applied in an agricultural setting, better equipping our crops to grow in resource-poor environments and to fight off dangerous pathogens. Indeed, the private sector has begun to invest in this approach. One strategy many companies are pursuing is a form of plant probiotic, which consists of preparations of beneficial microbes to be mixed with seeds at sowing and again once the seedlings germinate. Another approach is to use plant breeding to select for varieties that have enhanced symbiosis with the microbiota.
Many questions remain about the plant microbiome, however—not least of which is how thousands of years of cultivation have changed crops’ relationships with the soil biota. Using a cultivation-independent approach, my colleagues and I recently demonstrated that wild ancestors and modern varieties of barley (Hordeum vulgare) host distinct microbiotas.10 Likewise, Jos Raaijmakers of the Netherlands Institute of Ecology and colleagues last year identified a shift in the structure of the microbiome of modern and ancestral varieties of common bean (Phaseolus vulgaris); Bacteroidetes were more abundant in wild relatives, and their contribution to the community was progressively replaced by Actinobacteria and Alphaproteobacteria in the more domesticated plants.11
How do these differences translate to altered functionality of the microbiome? Thanks to the experience gained by Arabidopsis scientists, we are now in a position to address this question, and developing SynComs from crops will be an important step in the process.
Luckily, the field is motivated to do just that, as well as to define a road map to achieve the translational potential of the plant microbiome. In a few years, the plant microbiome manipulations may have moved from the lab to the field.
Davide Bulgarelli is a principal investigator at the University of Dundee in the U.K. His research aims at understanding the structure, function, and host control of the microbiome thriving at the root-soil interface.
References
- P. Bonfante, A. Genre, “Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective,” Trends Plant Sci, 13:492-98, 2008.
- D. Bulgarelli et al., “Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota,” Nature, 488:91-95, 2012.
- J. Edwards et al., “Structure, variation, and assembly of the root-associated microbiomes of rice,” PNAS, 112:E911-E920, 2015.
- D.L. Jones et al., “Carbon flow in the rhizosphere: carbon trading at the soil-root interface,” Plant Soil, 321:5-33, 2009.
- M.T. Agler et al., “Microbial hub taxa link host and abiotic factors to plant microbiome variation,” PLOS Biol, 14:e1002352, 2016.
- B. Niu et al., “Simplified and representative bacterial community of maize roots,” PNAS,114:E2450-E2459, 2017.
- R. Mendes et al., “Deciphering the rhizosphere microbiome for disease-suppressive bacteria,” Science, 332:1097-100, 2011.
- Y. Bai et al., “Functional overlap of the Arabidopsis leaf and root microbiota,” Nature, 528:364-69, 2015.
- G. Castrillo et al., “Root microbiota drive direct integration of phosphate stress and immunity,” Nature, 543:513-18, 2017.
- D. Bulgarelli et al., “Structure and function of the bacterial root microbiota in wild and domesticated barley,” Cell Host Microbe, 17:392-403, 2015.
- J.E. Pérez-Jaramillo et al., “Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits,” ISME J, 11:2244-57, 2017.
Source: www.the-scientist.com
Pasó por Oxford, Utah y París, da clases en Alicante, sigue aprendiendo inglés y tiene varios de los premios científicos más prestigiosos del mundo
“Si un joven quiere investigar fuera, bien por él; si quiere volver y no puede, eso sí que es una putada”
Hasta cuatro revistas rechazaron publicar su hallazgo. Hoy, gracias a su herramienta (CRISPR), se ha dado un paso gigantesco para acabar con enfermedades de origen genético
Tendría unos 10 años cuando se fue a su habitación de su casa en Elche, abrió la caja de gusanos de seda, cogió uno bien gordito, sacó la cuchilla y, zas, lo cortó por la mitad para ver qué tenía dentro.
-Al ver que no era sangre roja, me dije: “Este está enfermo”.
-¿Y qué hizo después, Francis?
-Coger otro.
El niño hizo exactamente lo mismo que con el anterior. Zas. Hubo un tercer gusano. Zas. Y un cuarto. Zas. Cinco minutos después, no quedaba uno entero en aquella cajallena de agujeros.
-Menuda escabechina.
-Sí. Pero entonces pude concluir: o todos los gusanos están enfermos o los gusanos no tienen la sangre roja.
Aquel Jack el Destripador de los anélidos hoy es el microbiólogo español más importante del mundo. Se llama Francisco Martínez Mojica, fuma Marlboro, lleva chupa de cuero y gafas que se oscurecen con el sol, dice joder y tío durante la entrevista, nos acerca servicialmente a donde queramos en su Passat de 135.000 kilómetros mientras escucha Talk FM para mejorar el inglés, ha ganado varios de los premios científicos más prestigiosos del planeta, da clases en la Universidad de Alicante y -tal y como dicen todas las quinielas internacionales cuando llega la fecha- es uno de los firmes candidatos a ganar el Nobel de Química o Medicina.
Eso más o menos lo sabe todo el mundo de Francis Mojica.
Lo que no sabe mucha gente es lo que les contaremos luego, eso que le ocurre cada vez que se acuerda de Josefina.
Ver entrevista
Fuente: www.elmundo.es
Non-tailed double-stranded DNA (dsDNA) viruses infect bacteria and dominate water samples from the world’s oceans. They have long escaped analysis because they have characteristics that standard tests can’t detect. However, scientists from MIT and elsewhere have now managed to isolate and study representatives of these elusive viruses.
Sourcer: http://www.sci-news.com/biology/autolykiviridae-05664.html
En junio del 2017 se realizó un llamado a estudiante de pregrado, postgrado o Socios de SOMICH, para crear el logo ALAM 2018. Dentro de las 6 propuestas recibidas, el logo ganador fue la propuesta realizada por el Sr. Felipe Andrés Serrano González, Lic. Ciencias Biológicas y Master Biología Celular y Molecular de la Pontificia Universidad Católica de Chile. Felicitaciones y te esperamos en ALAM2018!!!